GC Standard
The FDA, USP and EP all recognize the use of secondary standards and working standards that are established with reference to the corresponding primary standard. We offers pharmaceutical laboratories and manufacturers a broad range of secondary GC reference standards for various chromatographic and analytical applications. Our secondary GC standards give a convenient and cost-effective alternative to the pharmacopeias primary standards and allow laboratories to focus resources on their core activities rather than preparing internal working standards. Biosolve secondary GC reference standards are traceable to the current lots of the United States Pharmacopeia (USP); or to European Pharmacopoeia (EP) and British Pharmacopoeia (BP) if available. Most of our GC reference standards are completely synthetic and typically over 99.9% pure by GC-FID. Identifications determined by GC-MS, FTIR and 1H NMR and reported in the certificate of analysis of each manufactured lot.
| Image | Name | CAS No | Molecular Formula |
|---|---|---|---|
|
|
Methyl Ethyl Ketone
|
78-93-3
|
C4H8O
|
|
|
Methyl iso butyl ketone
|
[108-10-1]
|
C6H12O
|
|
|
Methyl-tert butyl ether
|
1634-04-04
|
C5H12O
|
|
|
n-Butanol
|
78-92-2
|
C₄H₁₀O
|
|
|
N-Hexane
|
110-54-3
|
C6H14
|
|
|
N-Pentane 99%
|
109-66-0
|
C5H12
|
|
|
N-Propanol
|
71-23-8
|
C₃H₈O
|
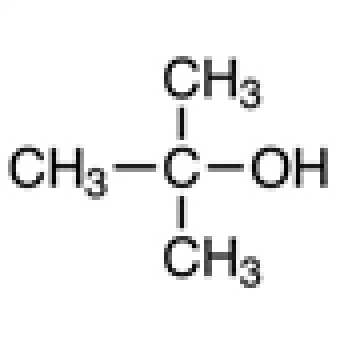 |
t-Butanol
|
75-65-0
|
C₄H₁₀O
|
|
|
Tetrahydrofurane
|
109-99-9
|
C4H8O
|
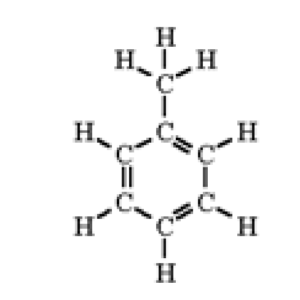 |
Toluene
|
108-88-3
|
C7H8
|
